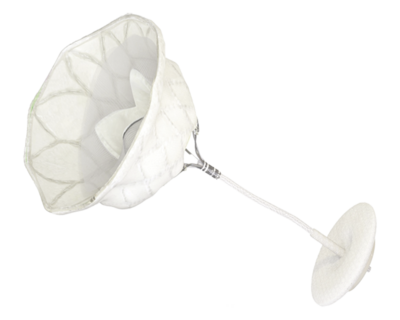
REPLACEMENT (TMVR)
Tendyne™ TMVR System is first-in-class technology designed to eliminate Mitral Regurgitation (MR),
offering selected patients with MR (≥ grade 3) a novel option for mitral valve replacement.
DISCOVER OUR LATEST CLINICAL OUTCOMES
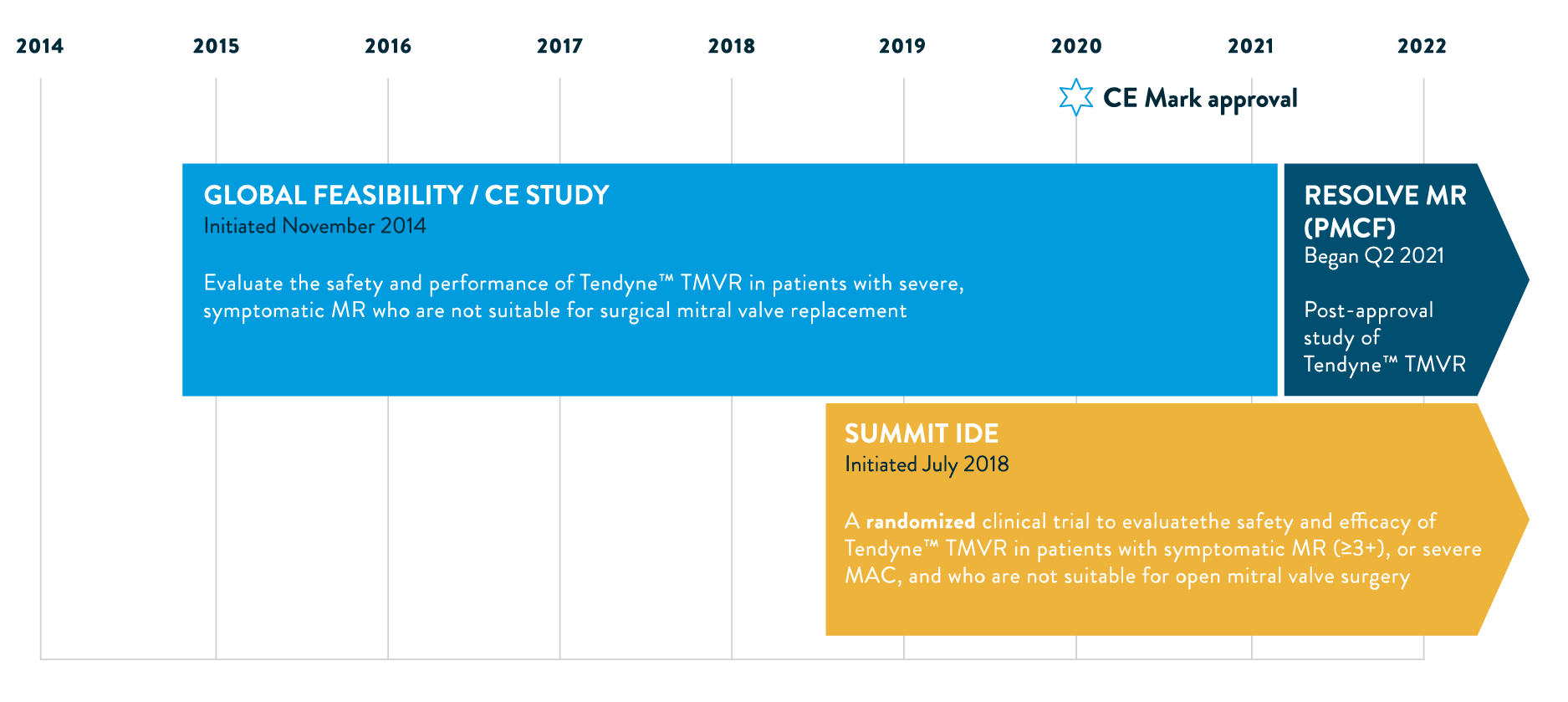
TENDYNE EXPANDED CLINICAL STUDY
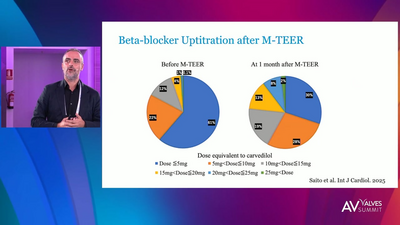
5 years outcomes presented at PCR LV 2025
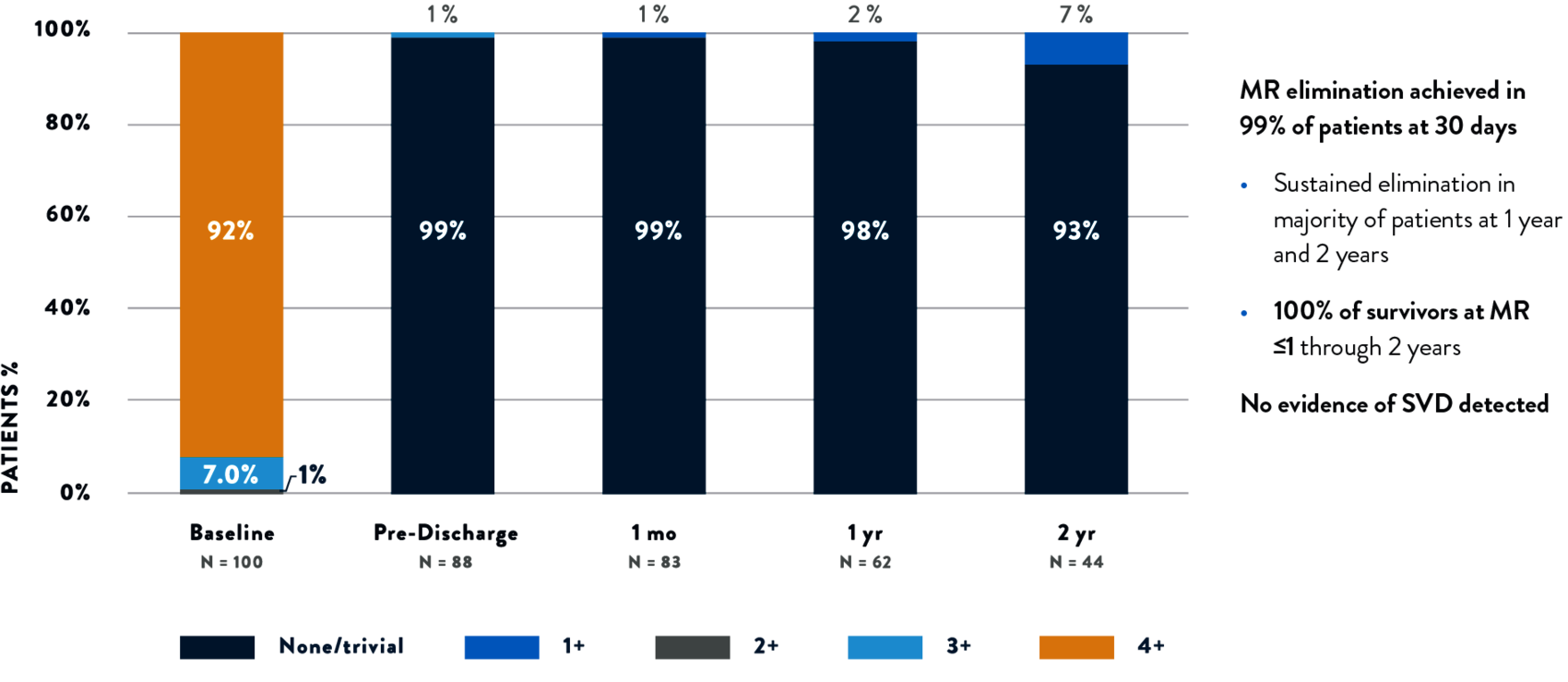
SUSTAINED MR ELIMINATION WITH TENDYNE™ TMVR
PREDICTABLE AND SUSTAINED CORRECTION OF MITRAL REGURGITATION THROUGH 2 YEARS
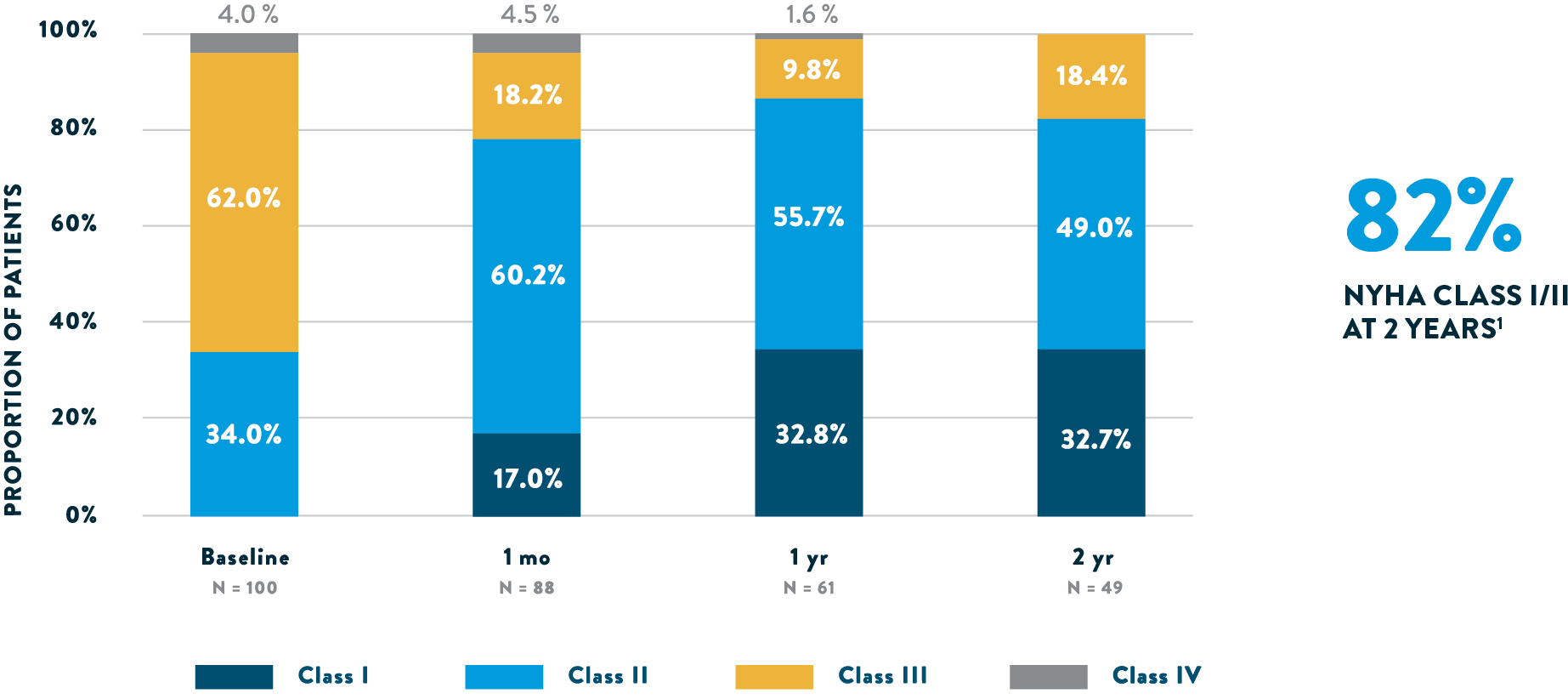
With Tendyne™ TMVR, patients can experience New York Heart Association (NYHA) class improvement. 81.6% of patients are in NYHA class I/II at 2 years versus 66.0% in class III/IV at baseline.1
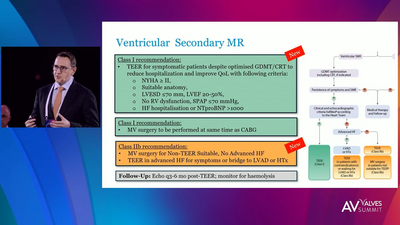
SYMPTOM IMPROVEMENT
NYHA Functional Class
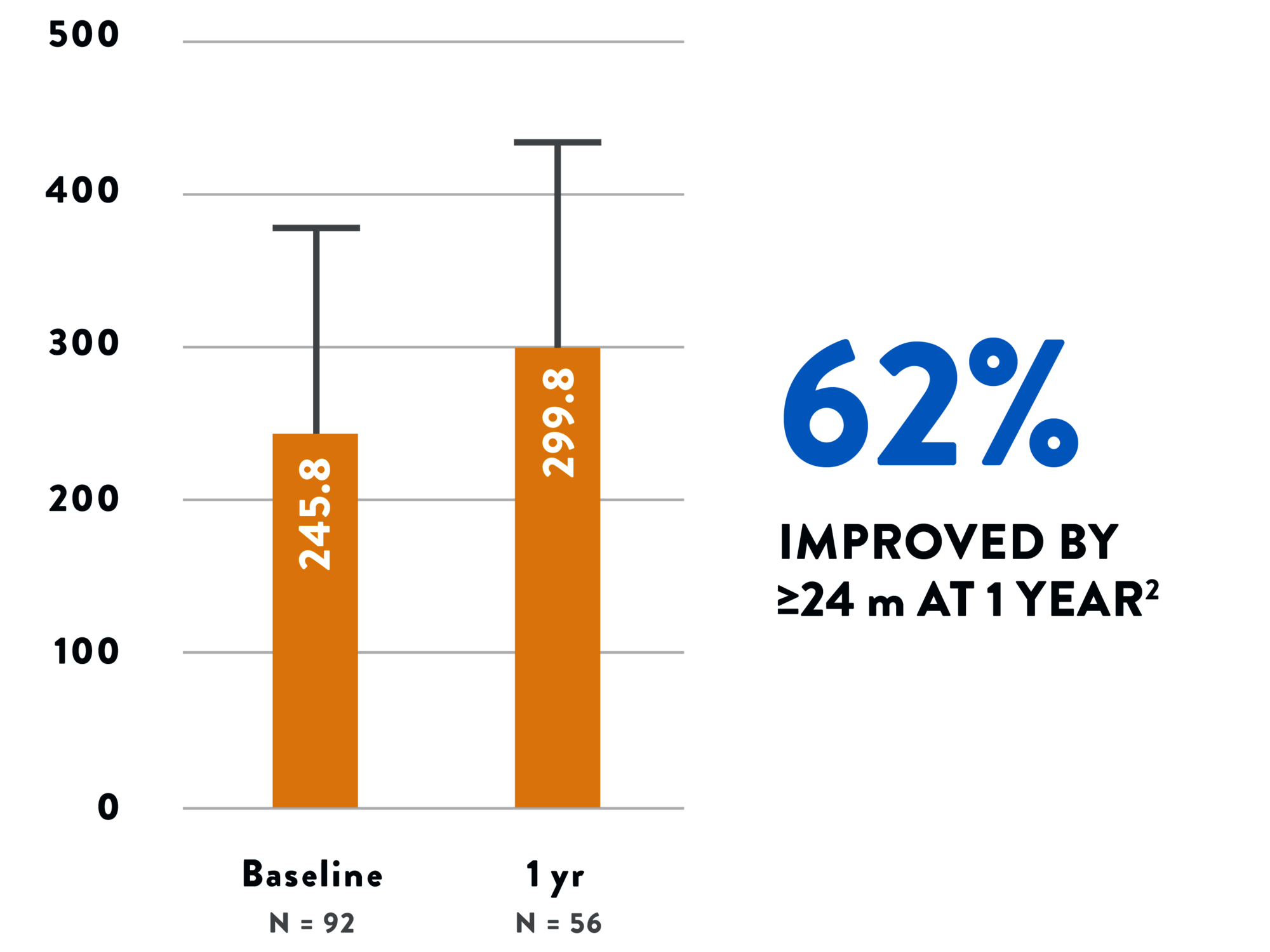
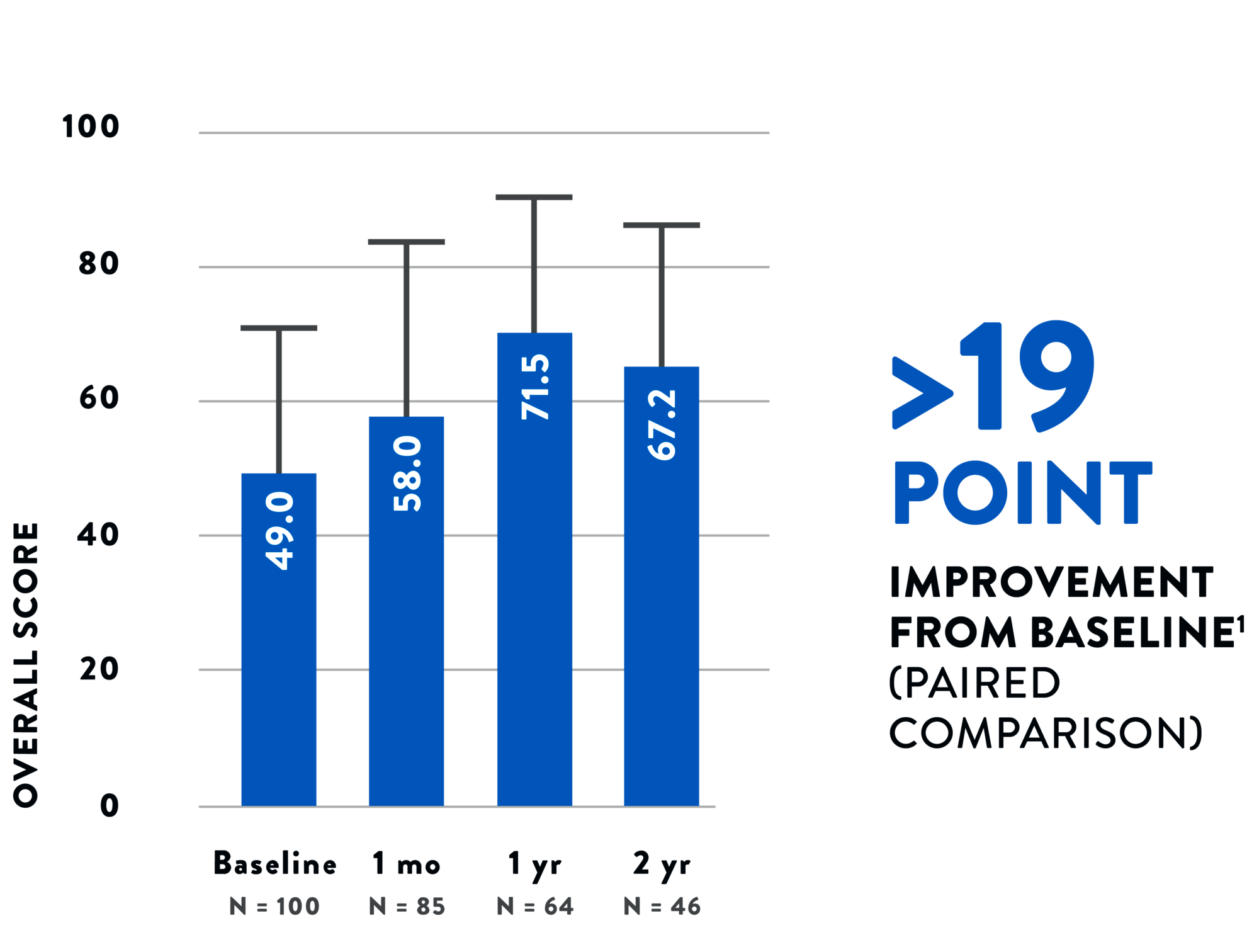
Tendyne™ TMVR improves your patients’ function and quality of life, with clinically significant improvement in a 6-minute walk test2 and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores.1 The 2-year all-cause mortality rate of 39% is an acceptable mid-term safety profile considering the advanced age and underlying comorbidities in this population.1
6-MINUTE WALK TEST2
QUALITY OF LIFE1 - KCCQ SCORES
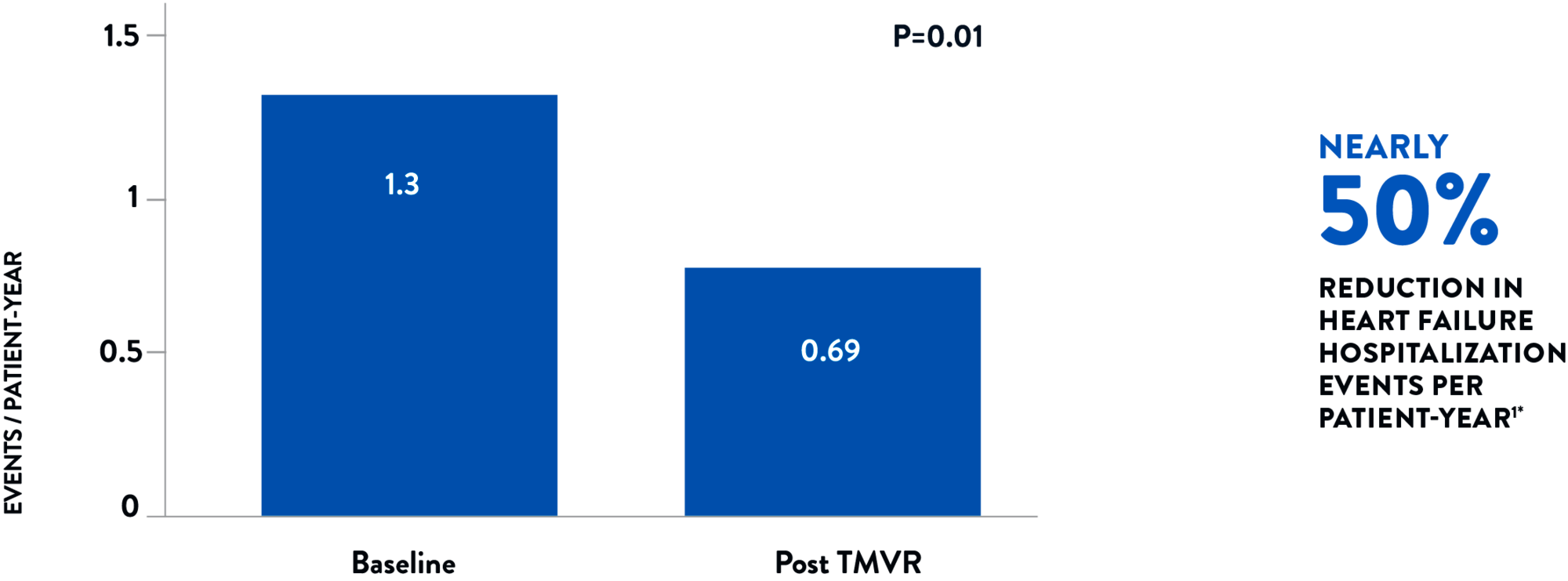
Assessed over a 6-month duration both before and after the procedure, heart failure hospitalizations were reduced by nearly 50% per patient-year.1
HEART FAILURE HOSPITALIZATION RATE
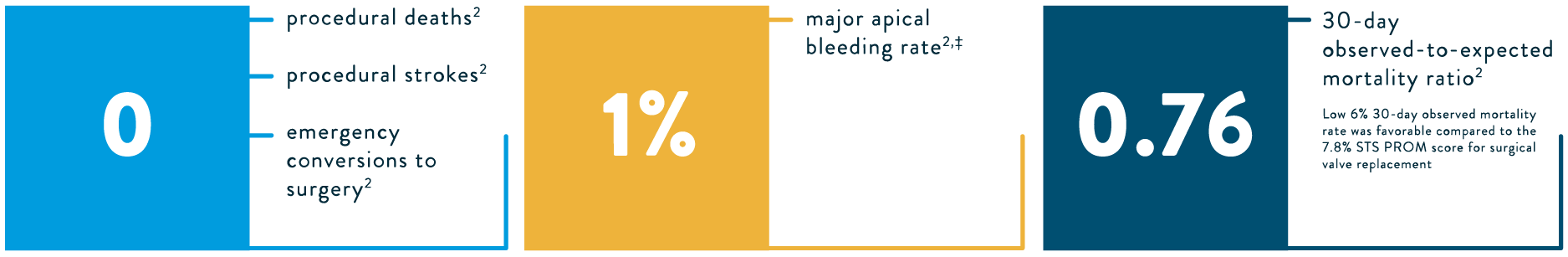
A technical success rate* of 96% was achieved, with low major adverse events2†:
ADVANCING TMVR THERAPY WITH CLINICAL EVIDENCE
LEARN MORE
Clinical case clubs | Educational tools | Hot topics
Expert opinions | Live & online discussions
TV
- Muller DWM, Sorajja P, Duncan A, et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J Am Coll Cardiol. 2021;78(19):1847–1859. doi.org/10.1016/j.jacc.2021.08.060.
- Sorajja P, Moat N, Badhwar V, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. 2019;73(11):1250–1260. doi.org/10.1016/j.jacc.2018.12.066.