HEART DEFECTS
Congenital heart defects are the most common type of birth defect. Defects that involve the wall or vessels of the heart include atrial septal defect (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). In certain situations, guidelines recommend surgery or transcatheter device closure to repair the defect and prevent complications.1
Designed for flexibility, our innovative duct occluder options conform to a variety of duct sizes while achieving complete patent ductus arteriosus (PDA) closure from a pulmonary or aortic approach.
PATENT DUCTUS ARTERIOSUS (PDA)
A patent ductus arteriosus (PDA) is an opening that abnormally remains open in the heart after birth, between the aorta and the pulmonary artery. A PDA can cause left-to-right shunting associated with bronchopulmonary dysplasia, pulmonary hypertension, necrotizing enterocolitis, intraventricular hemorrhage, retinopathy of prematurity, and renal impairment.2
From week 6 of fetal life until birth, the ductus is responsible for most of the right ventricular outflow. Normally, functional closure of the ductus arteriosus occurs by about 15 hours of life in healthy, full-term infants.
INCIDENCE AND MORTALITY WITH A PATENT DUCTUS ARTERIOSUS
- In full-term infants, PDA accounts for 5%–10% of all congenital heart disease3
- In preterm infants, the incidence of PDA can be up to 60%3
- Among neonates with a birth weight ≤ 2.6 pounds, 80% have PDA3
- Among preterm infants presenting with respiratory distress syndrome, about 80% have PDA3
- A PDA that fails to close by 2–3 days after birth may result in infant morbidity and mortality of up to 30%3
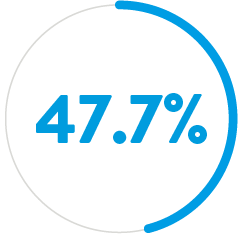
- Left untreated, a PDA carries mortality rates of:4
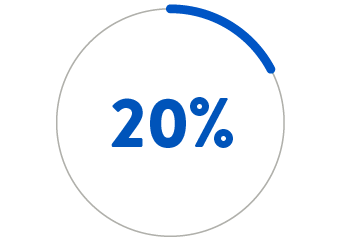
BY AGE 20
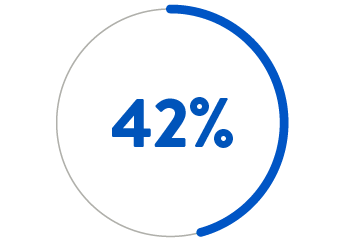
BY AGE 45
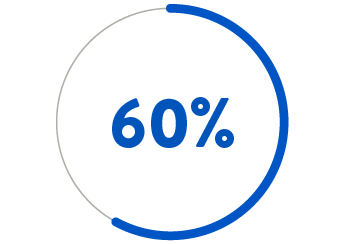
BY AGE 60
- Patients with a moderate left-to-right shunt may remain asymptomatic for years. However, historical series have shown that chronic volume overload may ultimately lead to severe complications:5
- Congestive heart failure
- Atrial arrhythmias
- Irreversible hypertensive pulmonary vascular disease
- Endarteritis
- In rare cases: ductus aneurysm or acute aortic dissection
Atrial Septal Defect (ASD)6

Ventricular Septal Defect (VSD)6

Pulmonary Stenosis6
If PDA is left untreated, the mortality rate is 20% by age 20 years, 42% by age 45, and 60% by age 60.4
Complications from an untreated PDA may include heart failure, renal dysfunction, necrotizing enterocolitis, intraventricular hemorrhage, diminished nutrition and growth, and potentially chronic lung disease.
Patients with a large PDA, when untreated, are at risk of developing Eisenmenger Syndrome, in which the usual left-to-right shunting reverses to a right-to-left shunt. At this point the PDA is irreversible, PDA closure is contraindicated, and lung transplantation may be the only option for long-term survival.
Neonatal presentation at physical examination
Assessment can reveal:
- Wide pulse pressure
- Bounding peripheral pulses
- Apnea (in neonates)
- Unexplained metabolic acidosis
- Hypotension and systemic hypoperfusion
Though not often observed in preterm infants, a murmur often obscures the second heart sound (S2). The murmur may be noticeable only during systole, or it may be a crescendo-decrescendo systolic murmur that extends into diastole.
Depending on the system affected by hypoperfusion, patients can also present with:
- Respiratory failure
- Cardiac hypertrophy
- Renal dysfunction
- Inability to tolerate feeding
- Necrotizing enterocolitis
Adult presentation at physical examination
Among adults, closure is indicated for patients with:
- Left atrial and/or left ventricular (LV) enlargement
- Pulmonary arterial hypertension
- Left-to-right shunt
- Prior endarteritis
WATCH ON-DEMAND:
TV
- Stout KK, Daniels CJ, Aboulhosn JA et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(14):e698–e800. doi.org/10.1161/CIR.0000000000000603.
- Sathanandam SK, Gutfinger D, O’Brien L et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv. 2020;96(6):1266–1276. doi.org/10.1002/ccd.28973.
- Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12(3):138–146. doi.org/10.5863/1551-6776-12.3.138.
- Kim LK. Patent ductus arteriosus (PDA). Medscape. emedicine.medscape.com/article/891096-overview. Accessed June 2023.
- Baruteau A-E, Hascoët S, Baruteau J et al. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014;107(2):122–132. doi.org/10.1016/j.acvd.2014.01.008.
- Tripathi A, Black GB, Park Y-M M et al. Prevalence and management of patent ductus arteriosus in a pediatric Medicaid cohort. Clin Cardiol. 2013;36(9):502–506. doi.org/10.1002/clc.22150.
- Philip R, Waller BR III, Agrawal V et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2016;87(2): 310–317. doi.org/10.1002/ccd.26287.
- Gillam-Krakauer M, Reese J. Diagnosis and management of patent ductus arteriosus. NeoReviews 2018;19(7):e394–e402. doi.org/10.1542/neo.19-7-e394.
- Chaudhary N, Filipov P, Bhutada A et al. Controversies in the management of patent ductus arteriosus in preterm infants. J Neonatal Biol. 2016;5(4):1000238. doi.org/10.4172/2167-0897.1000238. www.walshmedicalmedia.com/open-access/controversies-in-the-management-of-patent-ductus-arteriosus-in-preterminfants-2167-0897-1000238.pdf. Accessed June 2023.
- Nady ME, Amrousy DE, Salah N et al. Transcatheter versus surgical closure of patent ductus ateriosus in pediatric patients: A systematic review with meta-analysis. SM J Pediatr Surg. 2017;3(4):1054. www.jsmcentral.org/sm-pediatric-surgery/fulltext_smjps-v3-1054.pdf. Accessed June 2023.
- Zahn EM, Peck D, Phillips A et al. Transcatheter closure of patent ductus arteriosus in extremely premature newborns: Early results and midterm follow-up. JACC Cardiovasc Interv. 2016;9(23):2429–2437. doi.org/10.1016/j.jcin.2016.09.019.
- Van Overmeire B, Smets K, Lecoutere D et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343(10):674–681. doi.org/10.1056/NEJM200009073431001.
- Warnes CA, Williams RG, Bashore TM et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol. 2008;52(23):e143–e263. doi.org/10.1016/j.jacc.2008.10.001.
- Ashrafi AH, Levy VY. Management strategies for the preemie ductus. Curr Opin Cardiol. 2019;34(1):41–45. doi.org/10.1097/HCO.0000000000000580.
- Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125(5):1020–1030. doi.org/10.1542/peds.2009-3506.