PFO OCCLUDER
STRUCTURAL INTERVENTIONS
Effective patent foramen ovale (PFO) closure is made easier with the
Amplatzer™ Talisman™ PFO Occluder.1For patients who have experienced
a PFO-associated stroke, clinical trials have shown that they can benefit from
PFO closure.2–4This minimally invasive procedure significantly reduces the risk
of recurrent ischemic stroke and offers an excellent safety profile.
SWITCH TO THE REFERRAL VIEW For more information specific to neurology regarding PFO closure
THE SHIFT IN MY PRACTICE: PFO CLOSURE
Barry Love, MD and Lili Velickovic Ostojic, MD
Join leading experts as they discuss the latest clinical insights shaping real-world practice in reducing recurrent stroke.
Topics covered in this webinar include:
- Review of Current Guidelines and the Landscape for PFO Closure
- The Shift in My Practice: Insights from a Neurologist
- PFO Closure Now: Considerations from an Interventional Cardiologist
- Q&A
LEARN MORE:
THE LANDMARK RESPECT TRIAL2
STILL THE DEFINITIVE CLINICAL TRIAL ON PFO CLOSURE
STUDY DESIGN
The RESPECT trial is the largest trial ever conducted on PFO closure. This trial studied the Amplatzer™ PFO Occluder, on which the new Amplatzer™ Talisman™ device is based.
It stands apart from other PFO closure studies since it is the largest clinical trial with the most extensive follow-up, spanning 13 years. The 980 enrolled patients were followed for a median of 5.9 years. Researchers ultimately collected 5,810 patient-years of data—almost 2 times more than other PFO trials.
The trial was conducted at 69 centers across the U.S. and Canada and included patients on anticoagulation therapy— a real-world cross-section of patients— unlike other PFO trials, REDUCE3 and CLOSE.4


THE FINDINGS
The RESPECT trial offers conclusive evidence that using the Amplatzer™ PFO Occluder to close the PFO in patients with a PFO-associated stroke is more beneficial than medical therapy alone, reducing the risk of another stroke.
A meta-analysis on three randomized controlled trials on PFO closure confirmed that...“ PFO closure was superior to medical therapy for secondary prevention of stroke…with a more robust benefit when the Amplatzer™ PFO Occluder...was used.”6
FIND OUT MORE:
SUMMARY OF FINDINGS
EXCELLENT PROCEDURAL RESULTS
The RESPECT trial data reveal high rates of technical and procedural success and excellent closure with the study’s highly stringent criteria. At only 6 months, most patients also demonstrated freedom from shunt—both at rest and during Valsalva.2

Technical Success*

Procedural Success†

Effective Closure
(n9≤Bubbles)
EXCELLENT SAFETY2

Device Embolization

Device Erosion

Device Thrombus
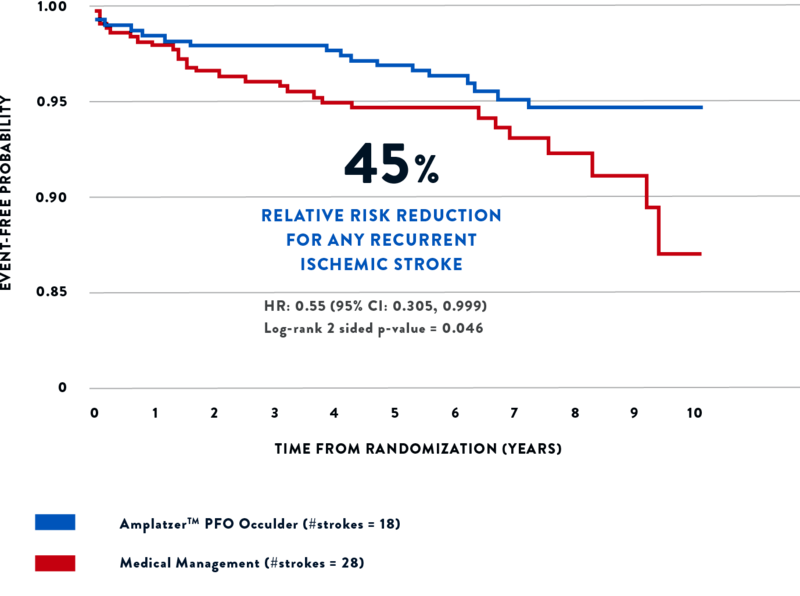
The RESPECT trial revealed a 62% relative risk reduction for recurrent cryptogenic ischemic stroke with the Amplatzer™ PFO Occluder versus medical therapy.2

The trial showed there was a 45% relative risk reduction for any recurrent ischemic stroke over nearly 6 years of follow-up, compared to medical therapy.2
The RESPECT trial also revealed low rates of serious atrial fibrillation (AF) with the Amplatzer™ device, consistent with medical therapy.2 AF rates were somewhat higher in the REDUCE trial,3 which used a different PFO closure device.
| RATE (PER 100 PATIENT YEARS) | RESPECT2 | REDUCE3 |
|---|---|---|
| Serious AF / Flutter* | 0.22 | 0.65 |
| Any AF / Flutter* | 0.76 | 1.90 |
COMPARISON OF PFO TRIALS: RESPECT1, REDUCE2 AND CLOSE3
| I I | RESPECT2 | REDUCE3 | CLOSE4 |
|---|---|---|---|
| Devices used | 100% Amplatzer™ PFO Occluder | 39% GORE HELEX, 61% GORE Cardioform | 51% Amplatzer™ PFO Occluder, 49% multiple approved |
| Patients | 980 | 664 | 473 |
| Follow-up patient-years | 5,810 (mean 5.9yrs) | 2,232 (mean 3.2yrs) | NR* (mean 5.4yrs) |
| Anticoagulant allowed in control group? | YES | NO | NO |
| Relative risk reduction | 62% (Recurrent ischemic stroke of unknown mechanism) | 77% (Recurrent ischemic stroke) | 97% (Recurrent ischemic stroke) |
| Effective closure | 94.2% Freedom from >9 bubbles (Evaluated after 6 months) | 94.5% Freedom from >25 bubbles (Evaluated after 9 months) | NR* |
Clinical case clubs | Educational tools | Hot topics
Expert opinions | Live & online discussions
TV
- Data on file at Abbott
- Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022–1032. doi.org/10.1056/NEJMoa1610057.
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033–1042. doi.org/10.1056/NEJMoa1707404.
- Mas J-L, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011–1021 and supplementary appendix. doi.org/10.1056/NEJMoa1705915.
- Mojadidi MK, Zaman MO, Elgendy IY, et al. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol. 2018;71(9):1035–1043. doi.org/10.1016/j.jacc.2017.12.059.
- Tests performed by and data on file at Abbott.
- Amplatzer™ Talisman™ PFO Occluder Instructions For Use.
- Satpathy R, Rodés-Cabau J, Thaler D, et al. Device closure of patent foramen ovale in patients >60 years with ischemic stroke: results from US Medicare beneficiaries. Presented at: ESOC 2024; May 15-17, 2024; Basel, Switzerland.