HEART DEFECTS
Congenital heart defects are the most common type of birth defect. Defects that involve the wall or vessels of the heart include atrial septal defect (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). In certain situations, guidelines recommend surgery or transcatheter device closure to repair the defect and prevent complications.1
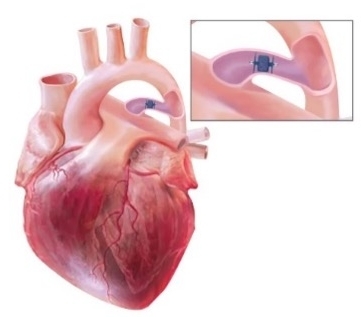
Designed for flexibility, our innovative duct occluder options conform to a variety of duct sizes while achieving complete patent ductus arteriosus (PDA) closure from a pulmonary or aortic approach.
The American College of Cardiology noted that many PDAs are now closed in infancy or childhood with catheter-based or surgical approaches. For those whose ductus remains patent in adulthood, catheter-based or surgical intervention consideration depends on the symptoms and physiological expression of the lesion.1
Yet in clinical practice there are inconsistencies in therapy strategies, especially for small and hemodynamically insignificant PDA. Some clinicians support closure to eliminate the lifelong risk of infective endarteritis, and others maintain that it is unnecessary.5
When an infant is asymptomatic or well controlled on medical therapy, closure treatment may be delayed until transcatheter therapy can be offered.
Adults with PDA are better suited for percutaneous closure due to high rates of success and low rates of complications.
Even when patients present with a small asymptomatic PDA, transcatheter device closure is a reasonable therapeutic approach.13
Early PDA closure can lead to rapid improvement in respiratory status and faster weaning off ventilator.
General PDA closure leads to proven benefits:
- Faster weaning off mechanical ventilator
- Oxygen supplementation
- Improved weight gain
- Faster decline in respiratory severity score (RSS)
- Potential avoidance of pulmonary hypertension (PHT)
Transcatheter closure with the Amplatzer Piccolo™ Occluder can provide more definitive closure than medication and is less invasive than surgery.
- Medication therapy has limited effectiveness (60–80%) and can have potential adverse effects in infants with PDA.14
- Surgical ligation is more invasive than the occluder implanting procedure and may lead to significant procedural complications.12,14,15
TV
- Stout KK, Daniels CJ, Aboulhosn JA et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(14):e698–e800. doi.org/10.1161/CIR.0000000000000603.
- Sathanandam SK, Gutfinger D, O’Brien L et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv. 2020;96(6):1266–1276. doi.org/10.1002/ccd.28973.
- Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12(3):138–146. doi.org/10.5863/1551-6776-12.3.138.
- Kim LK. Patent ductus arteriosus (PDA). Medscape. emedicine.medscape.com/article/891096-overview. Accessed June 2023.
- Baruteau A-E, Hascoët S, Baruteau J et al. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014;107(2):122–132. doi.org/10.1016/j.acvd.2014.01.008.
- Tripathi A, Black GB, Park Y-M M et al. Prevalence and management of patent ductus arteriosus in a pediatric Medicaid cohort. Clin Cardiol. 2013;36(9):502–506. doi.org/10.1002/clc.22150.
- Philip R, Waller BR III, Agrawal V et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2016;87(2): 310–317. doi.org/10.1002/ccd.26287.
- Gillam-Krakauer M, Reese J. Diagnosis and management of patent ductus arteriosus. NeoReviews 2018;19(7):e394–e402. doi.org/10.1542/neo.19-7-e394.
- Chaudhary N, Filipov P, Bhutada A et al. Controversies in the management of patent ductus arteriosus in preterm infants. J Neonatal Biol. 2016;5(4):1000238. doi.org/10.4172/2167-0897.1000238. www.walshmedicalmedia.com/open-access/controversies-in-the-management-of-patent-ductus-arteriosus-in-preterminfants-2167-0897-1000238.pdf. Accessed June 2023.
- Nady ME, Amrousy DE, Salah N et al. Transcatheter versus surgical closure of patent ductus ateriosus in pediatric patients: A systematic review with meta-analysis. SM J Pediatr Surg. 2017;3(4):1054. www.jsmcentral.org/sm-pediatric-surgery/fulltext_smjps-v3-1054.pdf. Accessed June 2023.
- Zahn EM, Peck D, Phillips A et al. Transcatheter closure of patent ductus arteriosus in extremely premature newborns: Early results and midterm follow-up. JACC Cardiovasc Interv. 2016;9(23):2429–2437. doi.org/10.1016/j.jcin.2016.09.019.
- Van Overmeire B, Smets K, Lecoutere D et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343(10):674–681. doi.org/10.1056/NEJM200009073431001.
- Warnes CA, Williams RG, Bashore TM et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol. 2008;52(23):e143–e263. doi.org/10.1016/j.jacc.2008.10.001.
- Ashrafi AH, Levy VY. Management strategies for the preemie ductus. Curr Opin Cardiol. 2019;34(1):41–45. doi.org/10.1097/HCO.0000000000000580.
- Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125(5):1020–1030. doi.org/10.1542/peds.2009-3506.