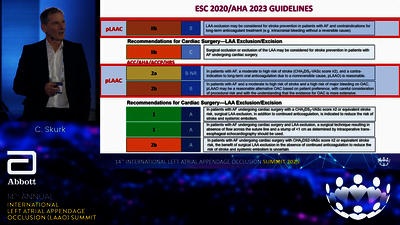
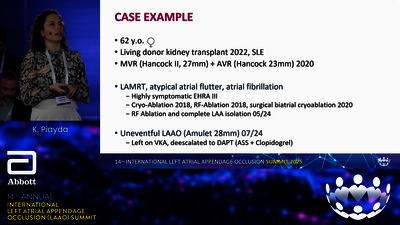
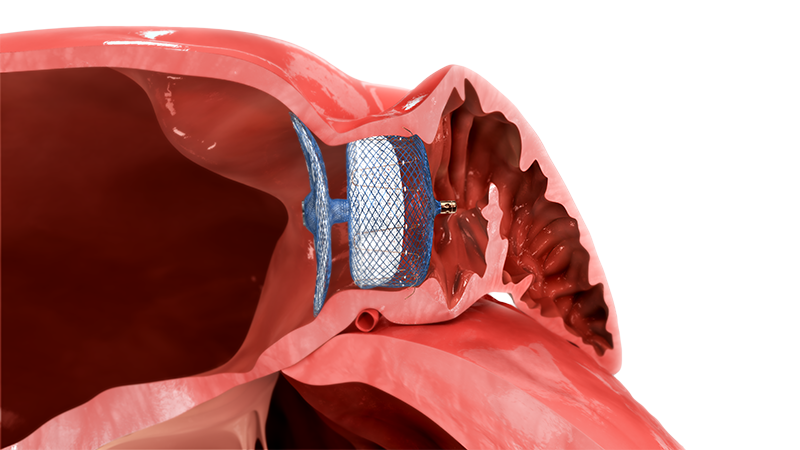
LAA OCCLUDER
STRUCTURAL INTERVENTIONS
Designed to treat patients with non-valvular atrial fibrillation (AF), who are at risk of ischemic stroke, the Amplatzer™ Amulet™ LAA Occluder offers complete closure of the left atrial appendage (LAA) and immediately eliminates the need for oral anticoagulants (OACs).1
SWITCH TO THE REFERRAL VIEW For more information specific to neurology regarding LAA Occlusion
KEY FEATURES
Innovative dual seal technology
The Amulet™ Occluder's innovative dual seal technology leverages a proven Amplatzer™ design that has been trusted for over 20 years.1
LEARN MORE:
More sizes for more anatomies
The Amulet™ Occluder offers a full range of sizes to ensure a precise fit across all LAA anatomies.

Physician education
Hear from physicians how the Amplatzer™ Amulet™ Occluder can benefit physicians and help patients live longer, fuller lives.
WATCH ON DEMAND:
TV
TV
- Amplatzer™ Amulet™ LAA Occluder Instructions for Use.
- Lakkireddy D, Thaler D, Ellis CR, et al. Amplatzer™ Amulet™ Left Atrial Appendage Occluder versus Watchman™ device for stroke prophylaxis (Amulet IDE): A randomized, controlled trial. Circulation. 2021; 144(19) :1543–1552. doi.org/10.1161/CIRCULATIONAHA.121.057063.
- Suradi HS, Hijazi ZM. Left atrial appendage closure: outcomes and challenges. Neth Heart J. 2017;25:143–151. doi.org/10.1007/s12471-016-0929-0.
- Kakkar AK, Mueller I, Bassand J-P, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE. 2013; 8(5):e63479. doi.org/10.1371/journal.pone.0063479.
- Baman JR, Mansour M, Heist EK, et al. Percutaneous left atrial appendage occlusion in the prevention of stroke in atrial fibrillation: a systematic review. Heart Failure Rev. 2018;23:191–208. doi.org/10.1007/s10741-018-9681-4.
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery; Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–429. doi.org/10.1093/eurheartj/ehq278. Erratum in: Eur Heart J. 2011;32(9):1172. PMID: 20802247.