TECH FOR LIFE
LEADING-EDGE CARDIOVASCULAR CARE
ESC Congress 2025
Shaping the Future of Structural Heart Therapies
ESC Congress 2025 delivered powerful momentum in TEER and TAVI, with key updates that are now shaping clinical practice. Dive into the highlights from this year’s event, including new guideline insights, Abbott’s expert-led symposium, late-breaking clinical data, indication extension, and exclusive interviews with thought leaders.
Explore on-demand educational tutorials designed to support your daily practice.
ESC/EACTS 2025 GUIDELINES
MITRAL & TRICUSPID TEER
TEER is a recommended alternative for patients with primary mitral regurgitation who are inoperable or at high surgical risk—supported by Class IIa, Level of Evidence (LoE) B. Early intervention is key, even in asymptomatic cases, especially when signs of cardiac damage like tricuspid regurgitation or left atrial enlargement are present.
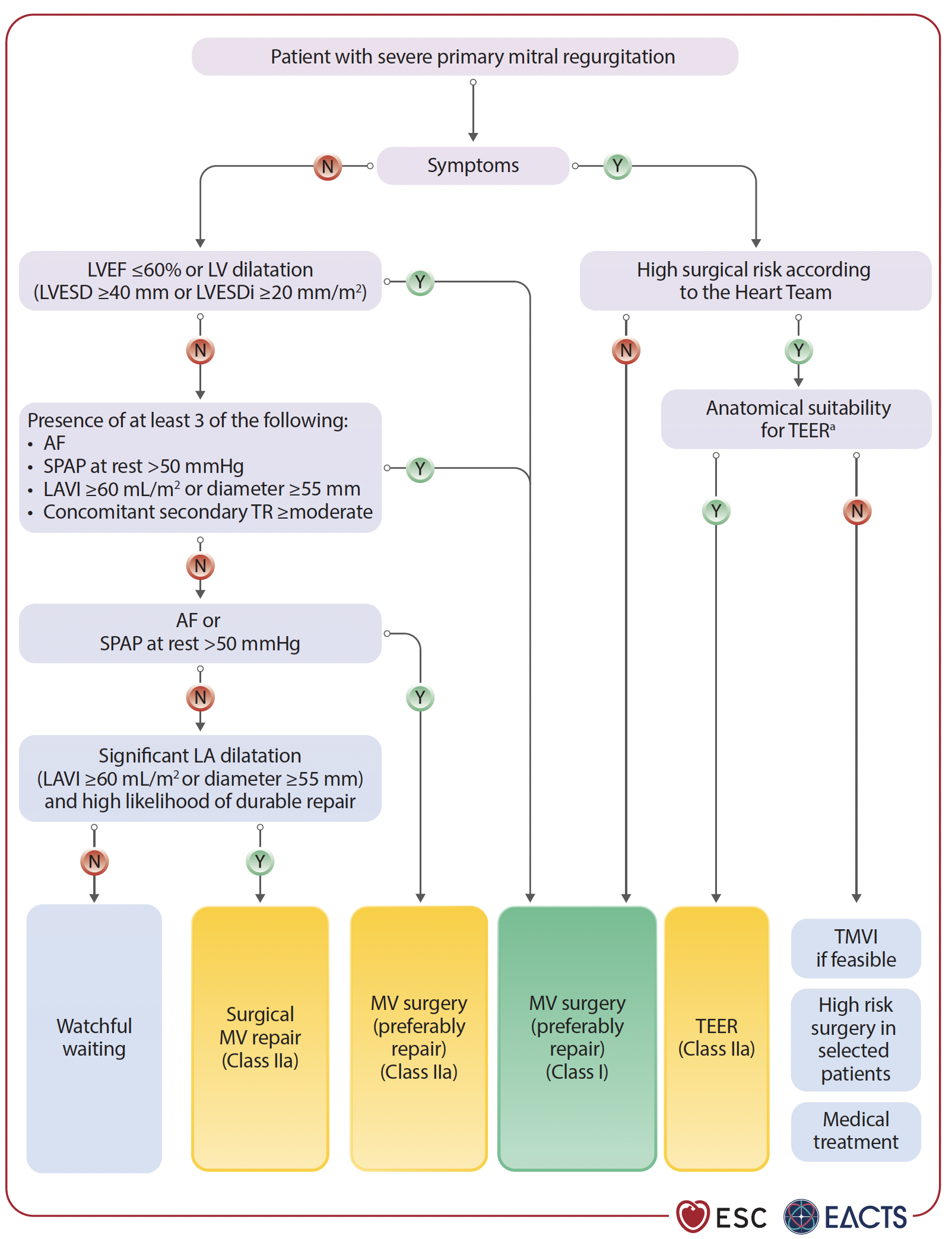
For secondary MR (SMR), guidelines now distinguish between ventricular and atrial types.
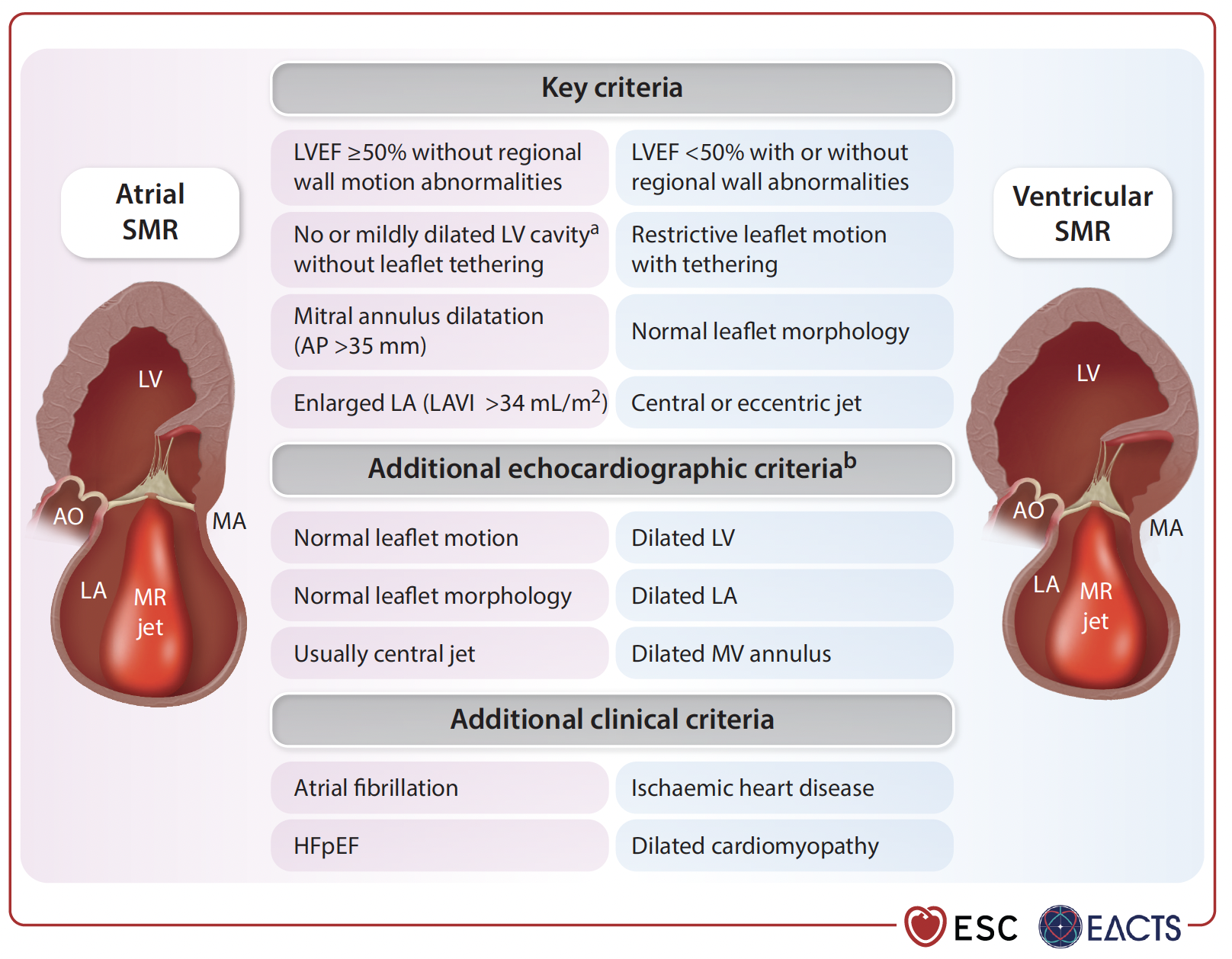
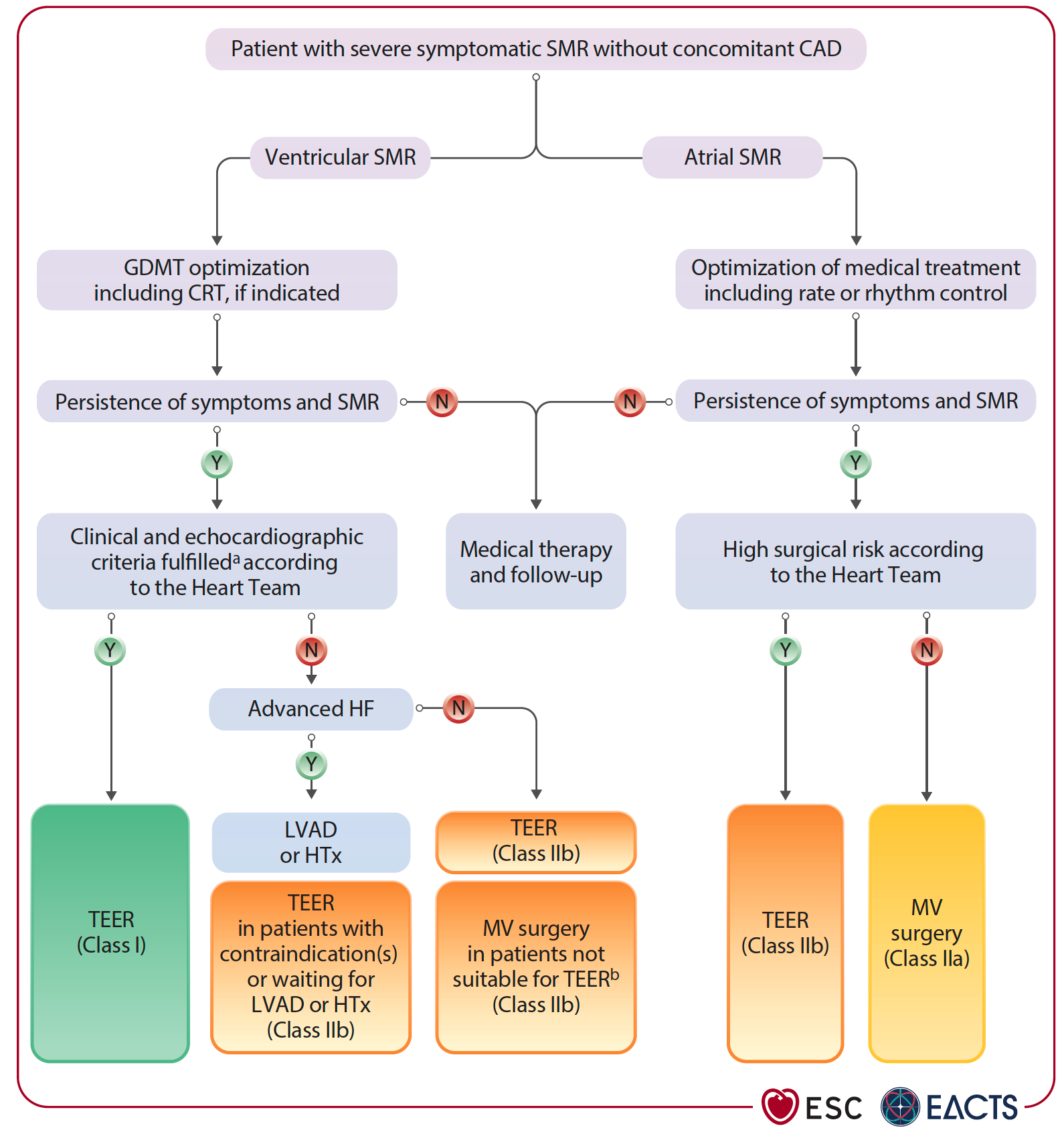
TEER is a Class I, LoE A recommendation for patients with ventricular SMR who remain symptomatic despite optimal medical treatment. In atrial SMR, surgery combined with AF ablation and LAAO may help, especially in patients with persistent symptoms and high stroke risk. TEER is also an option for those unsuitable for surgery, supported by a Class IIb, LoE B recommendation.
LEARN MORE:
The approach to mitral stenosis (MS) is evolving. Rheumatic MS is still treated with percutaneous mitral commissurotomy when anatomy allows, while surgery is reserved for complex cases. For degenerative MS linked to mitral annular calcification, guidelines now support transcatheter mitral valve implantation (TMVI) in select patients—reflecting growing expertise in this challenging area. TMVI has been deemed as Class IIb, LoE C.
LEARN MORE:
TR gains more interest in the ESC 2025 guidelines, with a Class Ia, LoE C recommendation for Heart Team evaluation. The focus is on early, systematic assessment using multimodality imaging. Transcatheter therapies are upgraded to Class IIa, LoE A for symptomatic severe TR—especially in high-risk patients—to enhance quality of life and support right ventricular remodeling.
LEARN MORE:
Watch the leading experts delving into the latest updates in ESC/EACTS VHD Guidelines

Optimizing Care Pathways in Mitral and Tricuspid Valve Disease
Translating the 2025 ESC/EACTS Guidelines into Practice
Watch V. Delgado, M. Metra and P. Lurz as they delve into the latest updates in ESC/EACTS VHD Guidelines. Explore the supporting clinical evidence behind the revised and newly introduced indications, and gain unique insights from their perspectives as experienced members of the Heart Team.
TAVI

Among the updated recommendations, TAVI is now indicated for anatomically suitable patients aged 70 years or older—lowered from the previous threshold of 75 years—regardless of surgical risk, provided the aortic valve is tricuspid. Pr. Borger said: “ There is an increasing amount of data from four randomized trials in patients 70 and over demonstrating very good early and mid-term outcomes and therefore we felt comfortable lowering the age limit’’.
The age cut-off shows, in the context of the VANTAGE data release supporting Navitor’s extended indication, the importance of selecting the right index valve preserving options for future interventions.
LEARN MORE:
SYMPOSIUM
Structural Heart Innovation Takes Center Stage at ESC
At this year’s ESC Congress, the Abbott Satellite Symposium ignited excitement around the future of structural heart therapies.
- Chaired by B. Aßmus and D. Capodanno, the session brought together leading voices in cardiology to spotlight the latest advancements
- R.S. von Bardeleben reviewed in depth the new VHD guidelines, setting the stage for clinical excellence.
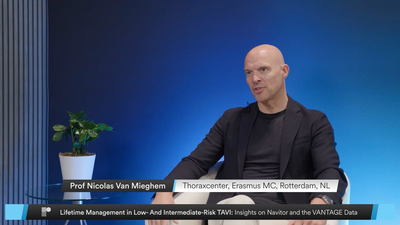
- N. Van Mieghem showcased the VANTAGE outcomes and introduced Navitor’s expanded indication for all risk patients, reinforcing its clinical impact.
- M. Keßler closed the session with a compelling look at the evolution of TEER and the release of G5, marking a new era in mitral valve therapy.
LATE BREAKER
VANTAGE STUDY CLINICAL OUTCOMES
WATCH THE LEADING EXPERTS SHARING THEIR PERSPECTIVES ON THE IMPACT OF TAVI FOR THE PATIENTS AT LOWER SURGICAL RISK.
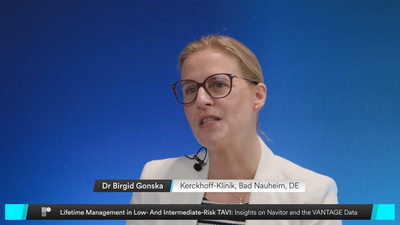
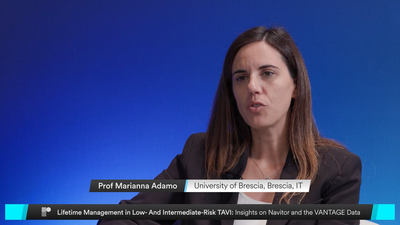
Watch N. Van Mieghem, G. Tarantini, and J. Wykrzykowska as they discuss expanding TAVI to patients at lower surgical risk and the clinical implications. Explore the VANTAGE study clinical outcomes, which confirm the Navitor™ TAVI System’s safety and effectiveness in low- and intermediate-risk patients. With a 97% success rate, low gradients, and no moderate or greater PVL at 30 days, Navitor demonstrates its versatility. At 12 months, the valve continues to provide significant benefits with low event rates. Together, these highlights position Navitor as a versatile platform for a broad range of patients.
THIRTY-DAY AND ONE-YEAR OUTCOMES OF NAVITOR TRANSCATHETER AORTIC VALVE IN LOW- OR INTERMEDIATE-RISK PATIENTS
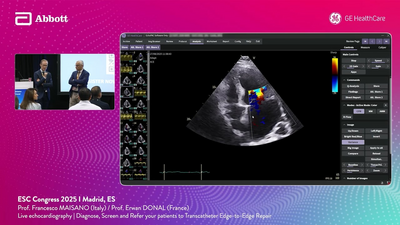
TUTORIALS
Explore 26 expert-led sessions from Abbott, covering the full spectrum of structural heart therapies. Gain clinical insights, exchange with peers, and discover real-world strategies to enhance patient care.