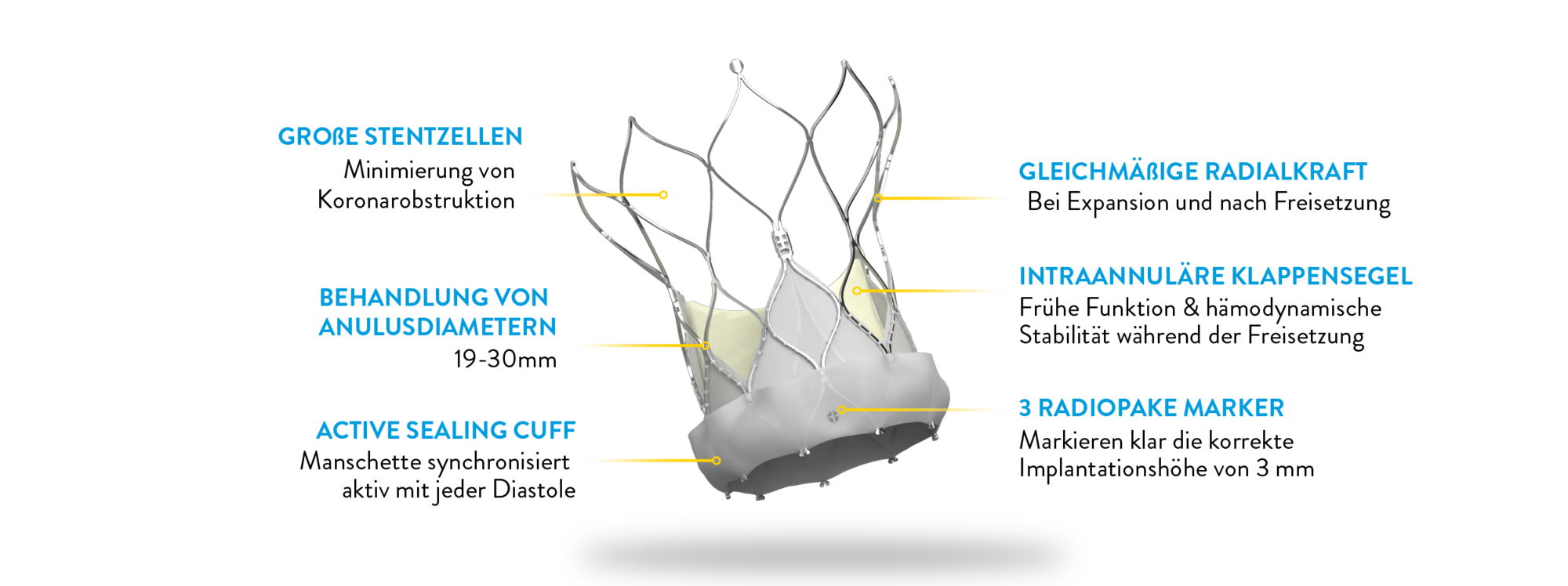
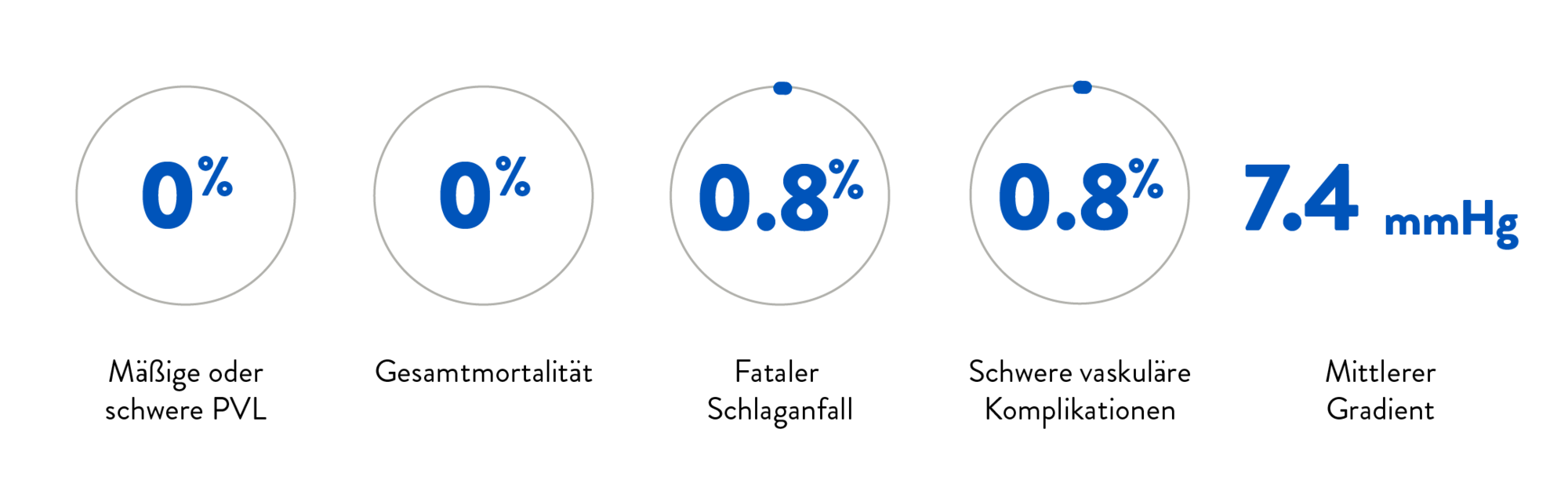
Durchdacht in jedem Detail.
Das Navitor-Transkatheter-Aortenklappen-Implantationssystem (TAVI-System) ist ein Vorreiter auf dem Gebiet des minimalinvasiven Herzklappenersatzes. Das Navitor TAVI-System wurde für eine stabile Positionierung, hervorragende Ergebnisse und für zukünftige Internventionen entwickelt und setzt einen neuen Standard auf dem Gebiet der TAVI.
SEHEN SIE SICH DIE LIVE-FÄLLE AN:
Live Case: EACTS Techno College Live Case aus dem Rigshospitalet Kopenhagen
Eine Klasse für sich
Das Navitor TAVI-System ist intuitiv, präzise und bietet eine exzellente Hämodynamik und Langlebigkeit für TAVI-Eingriffe. Das Design des Delivery Systems ermöglicht eine reibungslose eund optimierte Implantation und macht das Navitor System zu einer bevorzugten Wahl für Kliniker:innen und Patienten:innen gleichermaßen.
- Søndergaard L, Walton AS, Worthley SG, et al. Thirty-day and one-year outcomes of the Navitor transcatheter heart valve in patients with aortic stenosis: the prospective, multicentre, global PORTICO NG study. Eurointervention 2023;EIJ-D-22-01108. eurointervention.pcronline.com/article/thirty-day-and-one-year-outcomes-of-thenavitor-transcatheter-heart-valve-in-patients-with-aortic-stenosis-the-prospective-multicentre-global-portico-ng-study.
- Søndergaard, L. 30-day outcomes from a next generation TAVI device with an active sealing cuff. Presented at: EuroPCR conference; May 18–20, 2021.
- Pibarot P, Hahn RT, Weissman NJ, et al. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8(3):340–360.
doi.org/10.1016/j.jcmg.2015.01.008. - Frater RWM, Seifter E, Liao K, et al. Advances in Anticalcific and Antidegenerative Treatment of Heart Valve Bioprostheses. Austin, TX: Silent Partners Inc; 1997:105–114.
- Kelly SJ, Ogle, MF, Carlyle WC, et al. Biocompatibility and calcification of bioprosthetic heart valves. Society for biomaterials. Sixth World Biomaterials Congress Transaction. 2000;13534.
- Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation: efficacy and mechanisms. Circulation.
1997;95(2):479–488. doi.org/10.1161/01.CIR.95.2.479. - Vyavahare N, Hirsch D, Lerner E, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40(4):577–585.https://doi.org/10.1002/(SICI)1097-4636(19980615)40:4%3C577::AID-JBM9%3E3.0.CO;2-C.
- Gross JM. Calcification of bioprosthetic heart valves and its assessment. J Thorac Cardiovasc Surg. 2003;125:S6–8. doi.org/10.1067/mtc.2003.208.
- Tod TJ, Dove JS. The association of bound aldehyde content with bioprosthetic tissue calcification. J Mater Sci: Mater Med. 2016;27:8.
doi.org/10.1007/s10856-015-5623-z. - Meuris B, De Praetere H, Strasly M, et al. A novel tissue treatment to reduce mineralization of bovine pericardial heart valves. JTCVS. 2018;156(1):197–206. doi.org/10.1016/j.jtcvs.2018.01.099.
- Data on file at Abbott. 90664679
- Medtronic CoreValve Evolut‡ PRO Instructions for Use.
- Navitor™ TAVI System Instructions for Use.
- Boston Scientific ACURATE neo2‡ Instructions for Use.
- Boston Scientific iSleeve‡ Instructions for Use.
- Edwards Sapien‡ 3 Instructions for Use.
- Koehler Sapien‡ 3 eSheath OD BMRI 2015.
- Abbott data on file 90368819.
- Abbott data on file 90346620.
- Portico™ TAVI System Instructions for Use
‡Indicates a third-party trademark, which is the property of its respective owner.
Caution: These products are intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available)or at eifu.abbottvascular.com or at medical.abbott/manuals for more detailed information on Indications, Contraindications, Warnings, Precautions.
Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Abbott.