LATE-BREAKING DATA ON MITRAL AND TRICUSPID TEER
At ESC 2024, 3 investigator sponsored studies (ISS) for mitral and tricuspid valve repair therapies were presented as late-breaking data: the RESHAPE-HF2, MATTERHORN, Tri-FR trials. These clinical outcomes represent another milestone for Mitral and Tricuspid therapies demonstrating safety and efficacy with MitraClip™ and TriClip™ TEER.

RESHAPE-HF2
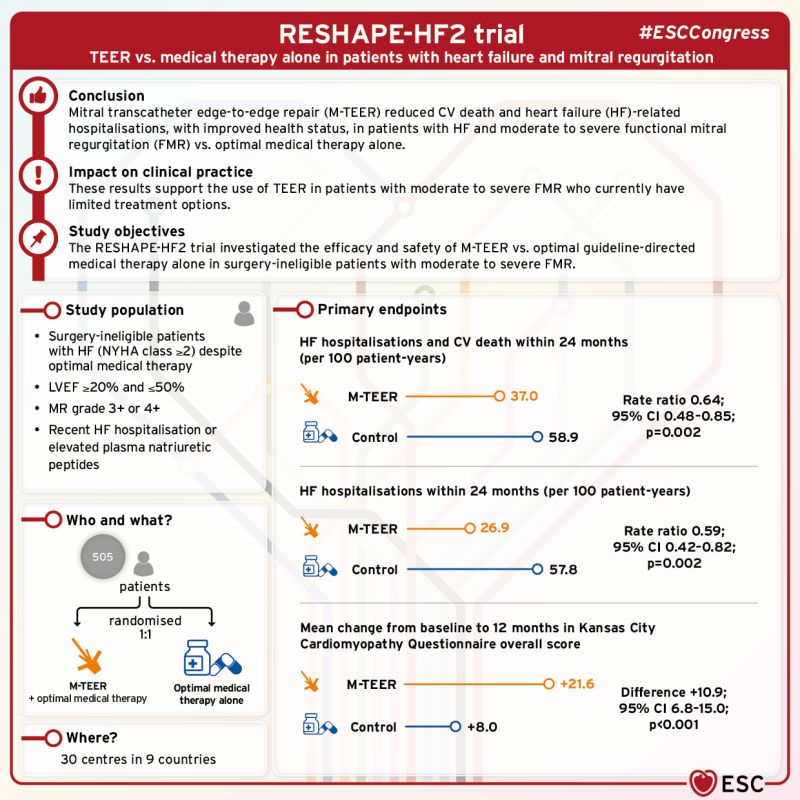
A second randomised clinical trial for MitraClip™ Transcatheter Edge-to-Edge Repair (TEER) in patients with heart failure and moderate-to-severe and severe secondary mitral regurgitation (SMR) that met its primary endpoint.1
Treatment with MitraClip™ TEER led to:
- 36% risk reduction in 24-month CV death and HFH; and 41% risk reduction in 24-month HFH1
- Significant improvement in quality of life +22 points at 12-month KCCQ-OS1
Results demonstrate that:
MitraClip™ TEER should be considered for patients with symptomatic heart failure and moderate-to-severe and severe SMR.1
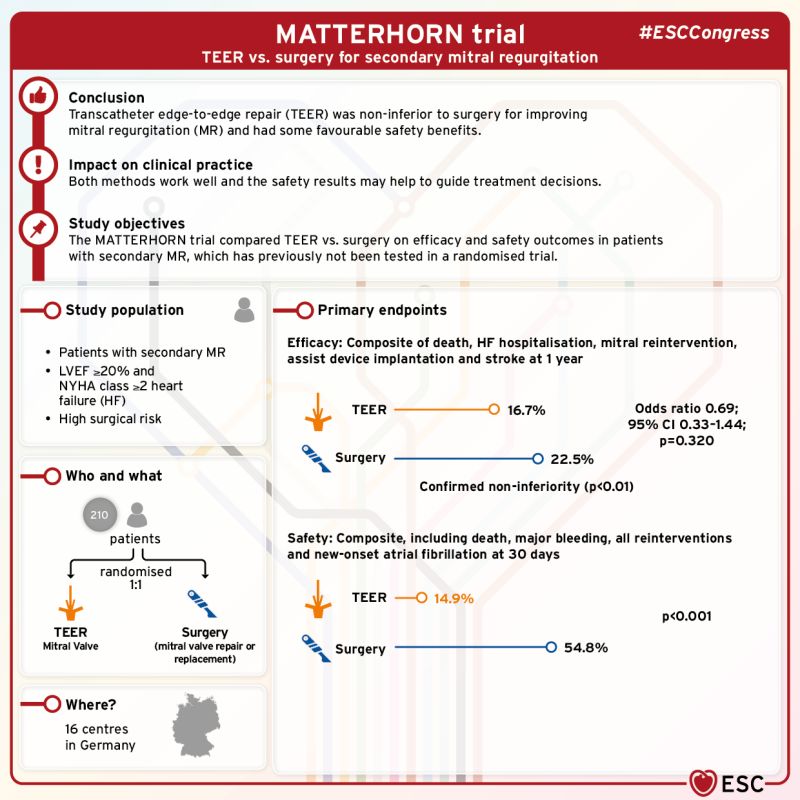
MATTERHORN
MATTERHORN results show MitraClip™ TEER was comparable to surgery among patients with SMR.2
Treatment with MitraClip™ TEER led to:
- A lower rate of MAEs than surgery at 30 days, through one year with 60% relative risk reduction2
- Comparable 1-year rates of death HFH, reintervention, LVAD or stroke with 17% MitraClip™ TEER vs 23% surgery2
Results demonstrate that:
MitraClip™ TEER achieved MR reduction comparable to surgery.2
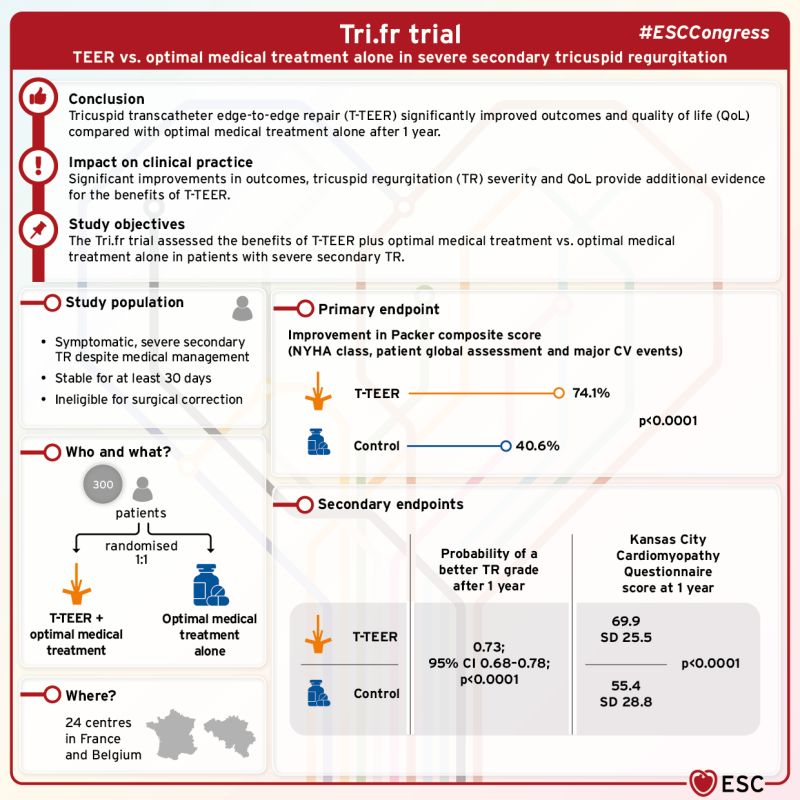
TRI.FR
TriClip™ TEER was superior to Medical Therapy alone.3
Treatment with TriClip™ TEER led to:
- Symptom improvements reported in 71% of patients treated with TriClip™ TEER vs. only 39% in the Medical Therapy group3
- 19% of patients experienced HFH or death in the control group vs. 13% in the TriClip™ TEER population3
- TriClip™ TEER confirms its exceptional safety profile with 0% peri-procedural death and 0.6% in hospital death3
- Multi-centered randomized evaluation of percutaneous Mitral Valve Repair (M-TEER) versus guideline-recommended medical therapy alone, in the treatment of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure – S. Anker et al, presented at ESC on August 31st 2024, London, UK.
- Multi-centered randomized evaluation of transcatheter versus surgical mitral valve repair in patients with heart failure and secondary mitral regurgitation – S. Baldus et al., presented at ESC on August 31st 2024 London, UK.
- Multicentric randomized evaluation of a Tricuspid valve Percutaneous Repair System (T-TEER) in the treatment of severe Tricuspid Regurgitation TRI.Fr – E. Donal et al., presented at ESC onAugust 31st 2024 London, UK.